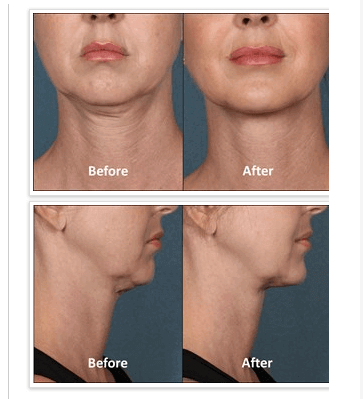
Introducing Kybella™
I was excited to hear that the FDA has approved a new injectable drug which will provide an addition to our non-surgical options for treating the dreaded“double chin”. Kybella™ is the first-in-class submental contouring injectable, which will offer physicians and their patients a non-surgical option to eliminate fat under the chin, resulting in a thinner and more contoured profile and jawline.”
How Kybella™ Works
Kybella™ is a formulation of a purified synthetic version of deoxycholic acid, a naturally-occurring molecule, found in the intestines, which is able to help break down fat in the body. When injected into the chin area, it destroys the fat cells present there, while also contouring the chin. This treatment is given under local anesthetic, with up to 50 injections spaced about one centimeter apart. As many as six treatments spaced at no less than one month apart, may be necessary to achieve your desired result. But most patients during the investigational trials were able to complete their treatment with 3-4 sessions. And unlike injectable fillers, once the treatment has been completed, re-treatment is not expected to be necessary.
When will it be available?
Kythera Biopharmaceuticals is planning to make Kybella™ available through plastic surgeons, facial plastic surgeons and dermatologists who have completed training on proper administration, in late summer. Pricing has not yet been announced, but we are expecting it to be in line with the prices of other injectable procedures.
And as always…
This is a promising non-surgical option for patients who are embarrassed by their double chins. It is important to remember, as with all injectable treatments, there will be people who are not good candidates. If you suffer from any medical conditions, you may need to discuss this with your doctor before having the injections to ensure that no negative interactions will be experienced as a result. READ KYBELLA™ PRESS RELEASE
Dr. G.